28+ Solution Definition Chemistry
Solution Definition Chemistry. When you dissolve a solid into liquid water, the structure is neither solid nor liquid, and we call it an aqueous solution. A solution is a system one comes across every day;

The solute is the substance that is dissolved in the solvent. The meaning of solution is something that is used or done to deal with and end a problem : A solution is usually defined as “ a homogeneous mixture of two or.
scie trepan bosch sichtschutz garten ideen metall rost salle de bain noir et rouge small upper arm tattoo male
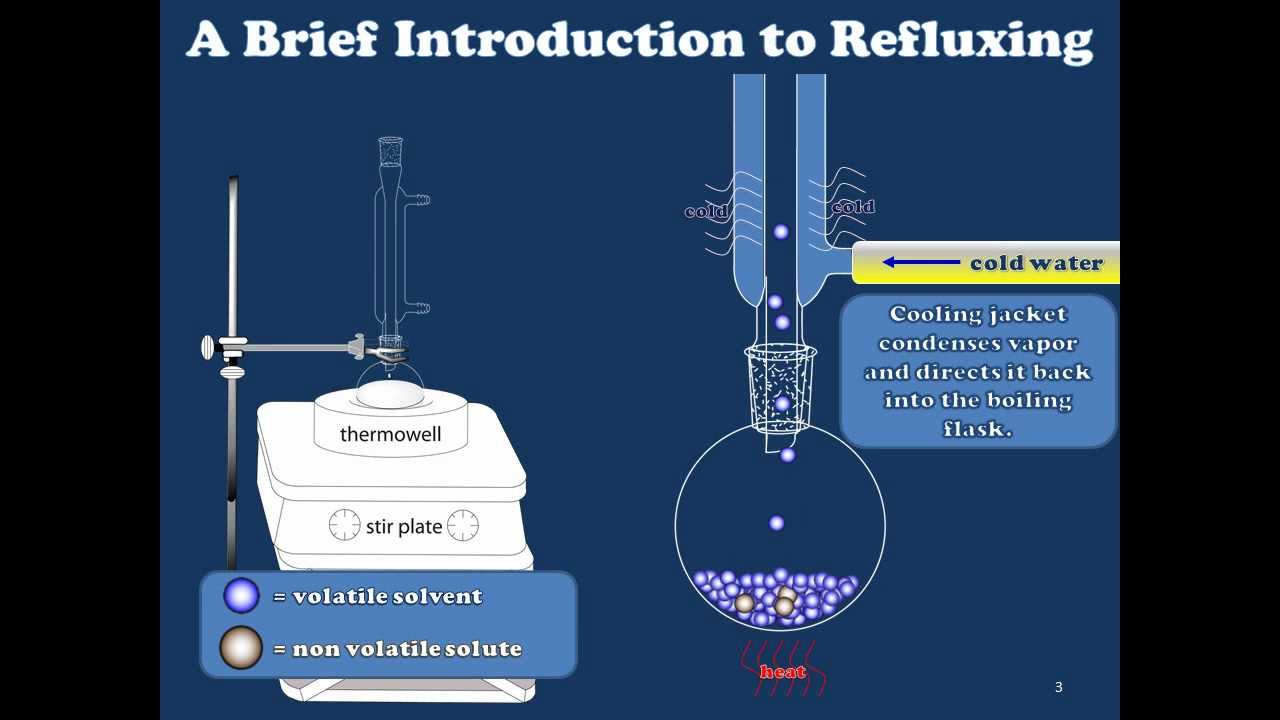
A Brief Introduction to Refluxing YouTube
A solutionis a homogeneous mixture of two substances—that is, it has the same distribution of particles throughout. Consolidates the latest research on nuclear fuel chemistry into Go here to learn more about mixtures. The solute is the substance that is dissolved in the solvent.

It is so well known that it needs no explanation. Buffer solution or simply called buffer in analytical chemistry or biology is a solution whose ph remains virtually unchanged upon the addition of small amounts of strong acids or bases. A solution is a homogeneous mixture of two substances—that is, it has the same distribution of particles throughout. In a.
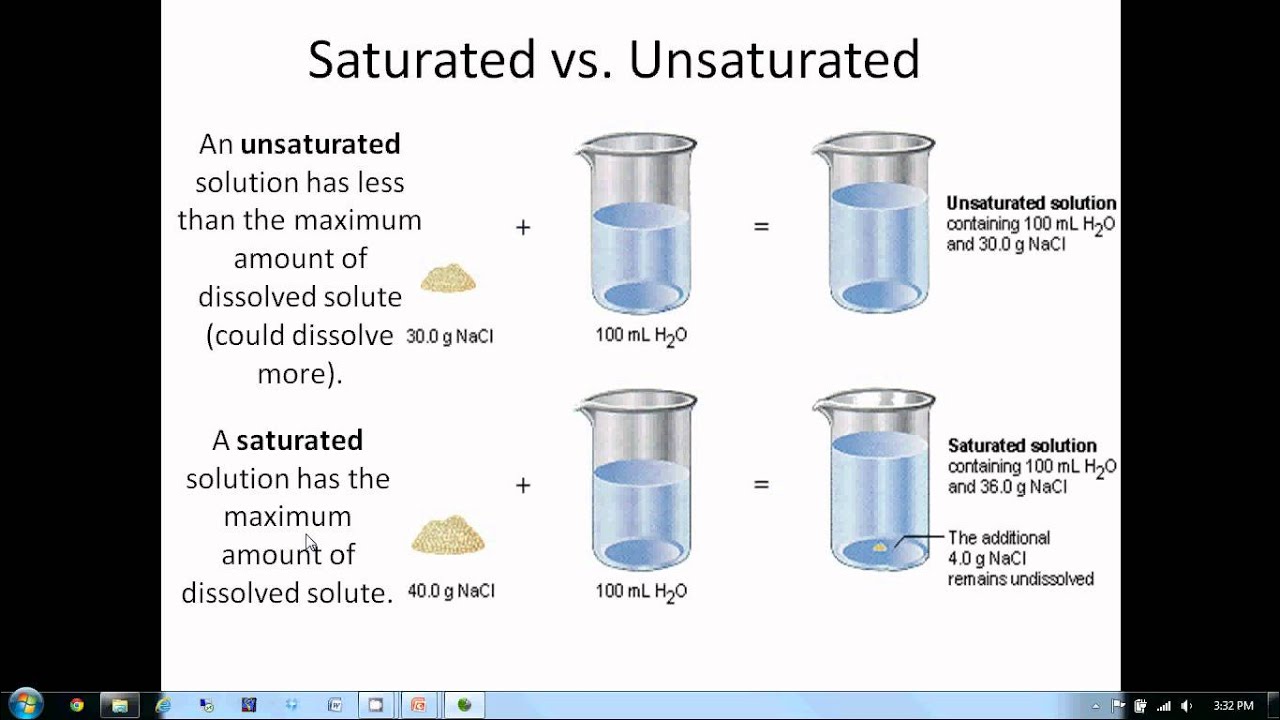
Go here to learn more about mixtures. It is so well known that it needs no explanation. The amount of solute that. It is uniform, or homogeneous, throughout the mixture. So, a solution has less structure than a solid yet more structure than a liquid.

Go here to learn more about mixtures. What is the chemical definition of a solution? Gaseous solutions, liquid solutions, and solid solutions. A solution has certain characteristics: These are equivalent to the three main phases used in chemistry.

A buffer solution may be made up of an acid and its conjugate base or a base and its conjugate acid. The particles of solute and solvent are molecules or ions, with one or more solvent molecules bound to each solute particle. A solution is a homogeneous mixture of two substances—that is, it has the same distribution of particles throughout..
Solution (chemistry), a mixture where one substance is dissolved in another. The solute is the substance that is dissolved in the solvent. A solution is a homogeneous mixture of one or more solutes dissolved in a solvent. A solution has certain characteristics: That’s why we consider it to be the 4th state of matter.

The term solution is commonly applied to the liquid state of matter, but solutions of gases and solids are possible. The particles of solute and solvent are molecules or ions, with one or more solvent molecules bound to each solute particle. However, it can be a gas, solid, or supercritical fluid. Go here to learn more about mixtures. Buffer solution.

Usually, a solvent is a liquid. The amount of solvent required to dissolve a solute depends on temperature and the presence of other substances in a sample. A solution is usually defined as “ a homogeneous mixture of two or. A solution is a homogeneous mixture of two substances—that is, it has the same distribution of particles throughout. A solution.

The solute is the substance that is dissolved in the solvent. A solution may exist in any phase. A solution is usually defined as “ a homogeneous mixture of two or. Usually, a solvent is a liquid. What is the chemical definition of a solution?